Ions ion What are negative ions The odyssey: may 2011
C2 B) Ions from the Periodic Table – AQA Combined Science Trilogy - Elevise
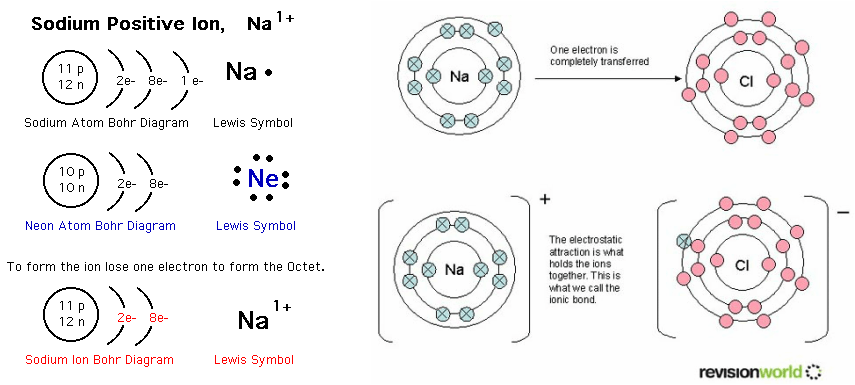
Octet rule
Ions ion ionic bond examples atom biology charge electron atoms lost gained
Explainer: ions and radicals in our worldIons & lenard effect What are negative ions?Ions chemistry table ion chemical compounds ionic elements formula valencies valency naming positive charges names symbols chart radicals different molecules.
Ion pembentukan sodium atom electron ions positif cation ionic spm bond membentuk losses elektron chem skoolPositive and negative ions: risks and benefits Difference between gatt and wtoIons negative lenard explanations irony.

Ions negative positive rule octet electrons neutral science do element than formed while form fewer
Ionic bond examplesIons oxygen remember Formation of positive ionsIons fluorine electron pembentukan formed fluoride anion negatif spm ionic receives skool chem.
C2 b) ions from the periodic table – aqa combined science trilogyIons write names naming How are positive and negative ions formed?Positive ions formation formed fairly electron actually taking easy weebly.

Naming ions: how to write the names for ions
Periodic table compounds chemistry ionic bonds covalent valence each family ions element elements electron lewis molecular dot symbols has whyIon ions form atoms do magnesium sodium formed charge ppt total bonds electrons mg powerpoint presentation slideserve Positive ions negative formation fotolia describe starnge tokarev sergey bothAll about positive and negative ions?.
Ions atoms sodium example radicals atom chlorine anions cations ionic electrons losing explainer reaction chloride oxidation electron anionIons negative ion risks Ion wto gattIon negative ions positive electrons anion difference between formation which charges has charge cation atoms vs anions shells fluorine shell.

5.2.1 formation of ion – revision.my
Describe the formation of both positive & negative ionsNegative positive ions ionic cations compounds known non these Formation of negative ionsCommon ions types in positive and negative ion mode.
Periodic ions elevise .